“I don’t want to be sort of a poodle dog when I’m out there, and a friendly sort of presence in people’s lives,” explained New York Times reporter David Carr in an October 2011 interview with Fresh Air host Terry Gross, “and then come back and do something that’s really mean or aggressive.”
“And if it’s gonna be a hard story, one of things I always say is: This is gonna be a really serious story, and I’m asking very serious questions. And it behooves you to think it through and really work on answering, and defending yourself, because this is not a friendly story. And if they don’t engage, I just tell them: Well, you know what? You better put the nut-cup on, because this is not gonna be pleasant for anyone.”
Two months later, I heard the interview as part of a year-end compilation of the show’s most memorable conversations. Carr’s comments struck a chord; just three weeks earlier, following the publication of “Perceived Predation Risk Reduces the Number of Offspring Songbirds Produce per Year” in Science, I was unable to get co-authors Liana Zanette and Michael Clinchy to “engage.”
Which I found both frustrating and puzzling.
Clearly, these two are eager to talk about domestic cats (see, for example, Clinchy’s comments in ScienceNOW and Zanette’s in the Winnipeg Free Press), even when their work has nothing at all to do with them (or, given the absurd methods employed, real-world predation in general). And here I am—pretty much all cats, all the time—reaching out, only to be snubbed.
During his Fresh Air interview, Carr refers to his October 23 column (“basically a screed,” he says), in which he took on big media—in particular, The Tribune Company and Gannett.
“I spent four days [in June] trying to get comments on Gannett [executive] bonuses, and on Sunday night they said, ‘We’re not going to comment on these bonuses.’ And I just said: Really? You’re a newspaper company. You’re a publicly held company. These bonuses are a matter of public record, and you have nothing to say about them? And I just found that appalling, and I think some of that was reflected in the piece.”
“Clearly,” added Carr, explaining the crux of his frustration, “they were living a life beyond consequence.”
Again, I’m reminded of Zanette and Clinchy. These two led a study in which contrived methods rendered the work nearly worthless—and then went on to misrepresent the study’s implications to the media. And what consequences will they face? None, I suspect. After all, the research did receive funding, and the resulting paper was published in a prestigious journal.
All of which paves the way for more of the same.
Not that Zanette and Clinchy are exceptional in this regard. Since launching this blog in April 2010, I’ve had numerous e-mail inquiries go unanswered. Scientists, journalists, officials of various agencies and organizations, etc.—people eager to get their message out, clearly, but unwilling to respond when that message is challenged. I’d always thought such scrutiny not only came with the territory, but was also welcome—a necessary tool for shaping better science, reporting, and policy.
Others apparently disagree. Among those with whom I have a decidedly one-way correspondence:
Melissa Miller, Wildlife Pathologist, California Department of Fish and Game
Miller was one of 14 co-authors to link the Type X strain of T. gondii—responsible for nearly three-quarters of sea otter infections, according to one study [1]—to wild felids (e.g., mountain lions and bobcats) rather than domestic cats.
“Three of the Type X-infected carnivores were wild felids (two mountain lions and a bobcat), but no domestic cats were Type X-positive. Examination of larger samples of wild and domestic felids will help clarify these initial findings. If Type X strains are detected more commonly from wild felids in subsequent studies, this could suggest that these animals are more important land-based sources of T. gondii for marine wildlife than are domestic cats.” [2]
Now, one needs to be very careful about making conclusions based on such small sample sizes. Nevertheless, given (1) the unprecedented (as far as I can tell) nature of these findings, and (2) the nature of the current “cat debate”—in which free-roaming cats are being vilified in both the scientific literature and mainstream media—this would certainly seem to be newsworthy.
And yet, just two paragraphs later, the paper goes into detail about the estimated mass of “feline fecal deposition” created by domestic cats in the communities near Estero Bay. Suddenly, the focus is back on domestic cats.
I asked her about this in July of 2010, but received no response.
(Nine months later, in a special section of the Spring issue of The Wildlife Professional, Miller and David Jessup (another of the 14 co-authors on the 2008 paper, and a colleague of Miller’s at the California Department of Fish and Game) were at it again, arguing simply, “the science points to cats.” [3])
Christine Stracey, Assistant professor of biology at Westminster College
“I thought the cats probably really hammered them when they were fledglings,” said Stracey, a former University of Florida doctoral student in a UF press release about her study of Northern mockingbirds, “but when they were in the nests, I didn’t really expect the cats to be a huge problem. But I was really wrong about that.”
Once again, the underlying science fails to live up to the dramatic press release. Dig into the details of Stracey’s study, and it becomes clear that she’s probably overestimating the strength of cats as urban predators. Perhaps considerably. In fact, her nest camera placement almost certainly biased her data.
In short, it seems Stracey observed predation by cats largely because she placed the cameras where the cats were.
“We need to think hard about the feral cat problem,” warns Stracey in the press release. But if, as she suggests, cats are a “huge problem,” then how to explain the fact—as Stracey notes in the very same press release—Northern mockingbirds have proven “able to not only live with us, but do really well living with us” [and our cats]? These birds are, as she puts it, “urban winners.”
I asked Stracey about all of this by way of e-mail, but received no response. My follow-up e-mail also went unanswered, but I did notice some Website traffic from the Salt Lake City area (where Westminster College is located) that same day. Coincidence? Could be.
The same goes for the traffic from Columbus, OH, following my e-mail to Amanda Rodewald, professor of wildlife ecology at Ohio State University.
“There are a lot of loud voices that deny cats are important predators of birds in our cities,” argued Rodewald (whose relationship to Stracey’s work remains a mystery) in the UF press release. “But this study shows clearly that cats were the dominant predator in this Florida system—and that wasn’t presumed, it was recorded on video, so it was fact.”
When I wrote to Rodewald, I identified myself as one of those “loud voices,” explaining that I wasn’t asking her to speak for Stracey, nor to defend the research. But, given her own research interest—and her obvious concern with Stracey’s work—perhaps she might be able to answer one question for me: What impact might we expect on the area’s Northern mockingbird populations if the cats were removed from the environment?
It was, apparently, one question too many.
Michael E. Grigg, National Institutes of Health
Like Zanette and Clinchy, Grigg, who serves as Chief of the Molecular Parasitology Unit at the National Institute of Allergy and Infectious Diseases (part of NIH), used a PR opportunity to misrepresent his work. “The most remarkable finding of our study,” notes Grigg in an NIH press release, “was the exacerbating role that [Sarcocystis] neurona appears to play in causing more severe disease symptoms in those animals that are also infected with T. gondii.”
So, the story is more complicated than is typically acknowledged—T. gondii may not be the culprit it’s so often made out to be.
But Grigg is still hanging his hat—in spite of his own findings—on simple environmental contamination:
“Identifying the threads that connect these parasites from wild and domestic land animals to marine mammals helps us to see ways that those threads might be cut… by, for example, managing feral cat and opossum populations, reducing run-off from urban areas near the coast, monitoring water quality and controlling erosion to prevent parasites from entering the marine food chain.”
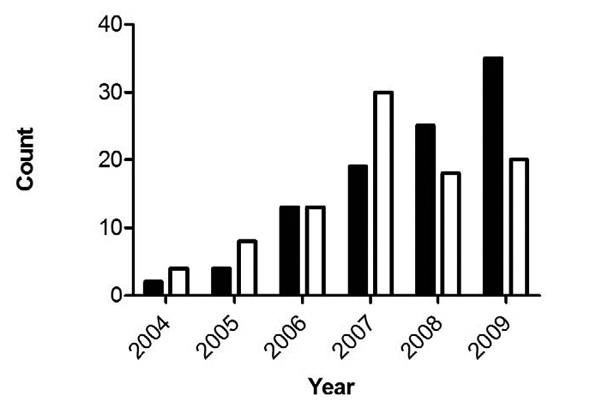
In fact, Grigg and his colleagues found that “T. gondii infections peaked in 2007 then declined relative to S. neurona.” [4] Could it be that free-roaming domestic cats—generally presumed to be the primary source of T. gondii contamination—are also on the decline? TNR opponents don’t seem to think so.
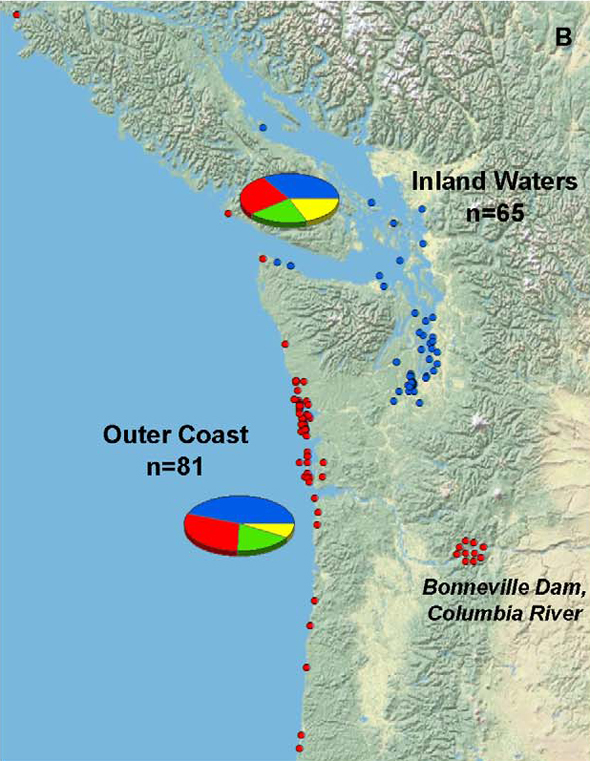
And the researchers observed that infection rates associated with inland waters were no greater than in mammals found along the outer coast. Again, this raises serious questions about the role of domestic cats (which, one would presume, are more numerous inland). As do the numerous studies pointing to sources other than environmental contamination [5–7]—vertical transmission, [5] for example, and possibly ticks [8]—none of which Grigg and his colleagues acknowledge.
Then, of course, there are Grigg’s proposed solutions—first and foremost: “managing feral cat and opossum populations.” Even setting aside for the moment the numerous hurdles (e.g., ethical, economic, etc.) involved with the mass removal/reduction/eradication of these animals, what impact could we realistically expect in terms of T. gondii and/or S. neurona infections in marine mammals? (And what other consequences would we then face?)
I assume Grigg has given the subject considerable thought, but—so far, anyhow—he’s been unwilling to share those thoughts with me.
Steve Klett, Crocodile Lake National Wildlife Refuge Manager
I first ran across Klett’s name in the Florida Keys National Wildlife Refuges Complex Integrated Predator Management Plan/Draft Environmental Assessment, where he was cited as the source for the claim that “cats accounted for 77 percent of the mortality during a recent re-introduction of the Key Largo woodrat.” [9] If, as USFWS has suggested, there are only about 500 woodrats in the wild, [10] why not disclose precisely how many were involved in this “recent re-introduction”? Seventy-seven percent out of how many?
Thirteen, as far as I’ve been able to determine. That’s how many were apparently released in November 2010—following the release of 14 others in February. And, according to attorney and Endangered Species Act blogger Keith Rizzardi, 13 more were released in April 2011.
I e-mailed Klett in July, asking him to clarify that 77 percent figure (which, let’s face it: does a far better job of fanning the flames of the witch-hunt for feral cats than, say, “10 out of 13” does), but never heard back.
Klett retired in December, but Chad Anderson, USFWS biologist at the refuge, assures me that I can “look forward to that [77 percent figure] going from a personal comm. quote to a referenced white paper in the final IPM plan.”
I’m not holding my breath.
Timothy O’Hara, Reporter for the Key West Citizen
In an August 30, 2011 story for the Key West Citizen, Timothy O’Hara writes: “Research indicates that cat predation accounts for 50 percent to 77 percent of the deaths of Lower Keys marsh rabbits and Key Largo woodrats.”
One-half to three-quarters? Really?
Actually, no.
That 77 percent, of course, comes from Klett’s “personal communication”—and seems to reflect the 10 mortalities described above. (Another question I asked Klett was how they could be sure that cats were the culprits. It’s been suggested by a volunteer involved with the re-introductions that the evidence comes from camera traps on the refuge, but I know of no such information coming from refuge officials.)
The 50 percent figure, I’m quite sure, can be traced to Elizabeth Forys’ PhD work, done in the early 1990s on Navy-owned land on Boca Chica and Saddlebush Key. Forys found that 13 of 24 marsh rabbits monitored over the course of her two-and-a-half year study were killed by cats. [11] (USFWS misrepresents this, too, in its Predator Management Plan, once again omitting the number of mortalities: “Free-roaming domestic cat predation accounted for 50 percent of adult Lower Keys marsh rabbit mortality during radio telemetry studies…” [9])
Turns out, O’Hara wasn’t interested in being fact-checked; he never replied to my e-mail.
(If O’Hara is interested in real journalism, he might consider an investigative piece about how USFWS routinely plays misleads the public to whom they are ostensibly accountable.)
Darin Schroeder, ABC’s Vice President for Conservation Advocacy
In October, Schroeder sent a letter (PDF) to the mayors of the 50 largest cities in the country, urging them “to oppose Trap-Neuter-Re-abandon (TNR) programs and the outdoor feeding of cats as a feral cat management option.” In it, he trots out the usual laundry list of misleading complaints: predation, rabies, vague threats regarding the possible implications of the Endangered Species Act and the Migratory Bird Treaty Act, etc.
When I wrote to Schroeder, I made it clear that we need not get into all of this. I just wanted him to explain how feeding bans and policy directives opposing TNR would, as ABC suggests in its November 9 media release, “stop the spread of feral cats.” After all, common sense—and science—tells us that such policies (assuming they could be enforced, of course) would only drive population numbers upward. If, as Schroeder claims, there are “well-documented impacts of cat predation on wildlife,” how could an increase in the population of cats possibly be a benefit?
That was nearly two months ago now; I’m still waiting for Schroeder to connect the dots for me.
• • •
At the risk of stating the obvious, I’m not David Carr. And this is not the New York Times. I suppose my inquiries are easily ignored—coming, as they do, from an “outsider” whose blog has just 330-some subscribers. On the other hand—and not to put too fine a point on it—I’m asking the kinds of questions these people should be asked, by their colleagues, the press, and, in the case of the non-profits like the American Bird Conservancy, their donors.
That seems to be changing though—which means these folks had better get to work on better responses (or, as Carr suggests, put the nut-cup on). In the meantime, the fact that they refuse to engage speaks volumes.
Literature Cited
1. Conrad, P.A., et al., “Transmission of Toxoplasma: Clues from the study of sea otters as sentinels of Toxoplasma gondii flow into the marine environment.” International Journal for Parasitology. 2005. 35(11-12): p. 1155-1168. http://www.sciencedirect.com/science/article/B6T7F-4GWC8KV-2/2/2845abdbb0fd82c37b952f18ce9d0a5f
2. Miller, M.A., et al., “Type X Toxoplasma gondii in a wild mussel and terrestrial carnivores from coastal California: New linkages between terrestrial mammals, runoff and toxoplasmosis of sea otters.” International Journal for Parasitology. 2008. 38(11): p. 1319-1328. http://www.sciencedirect.com/science/article/B6T7F-4RXJYTT-2/2/32d387fa3048882d7bd91083e7566117
3. Jessup, D.A. and Miller, M.A., “The Trickle-Down Effect.” The Wildlife Professional. 2011. 5(1): p. 62–64.
4. Gibson, A.K., et al., “Polyparasitism Is Associated with Increased Disease Severity in Toxoplasma gondii-Infected Marine Sentinel Species.” PLoS Neglected Tropical Diseases. 2011. 5(5): p. e1142. http://dx.doi.org/10.1371%2Fjournal.pntd.0001142
5. Hide, G., et al., “Evidence for high levels of vertical transmission in Toxoplasma gondii.” Parasitology. 2009. 136(Special Issue 14): p. 1877-1885. http://dx.doi.org/10.1017/S0031182009990941
6. Prestrud, K.W., et al., “Serosurvey for Toxoplasma gondii in arctic foxes and possible sources of infection in the high Arctic of Svalbard.” Veterinary Parasitology. 2007. 150(1–2): p. 6–12. http://www.sciencedirect.com/science/article/B6TD7-4PYR4P2-2/2/fcc91fcf1d1426cd1b750bd3840bdb31
7. Oksanen, A., et al., “Prevalence of Antibodies Against Toxoplasma gondii in Polar Bears (Ursus maritimus) From Svalbard and East Greenland.” Journal of Parasitology. 2009. 95(1): p. 89–94. http://dx.doi.org/10.1645/GE-1590.1
8. Sroka, J., Szymańska, J., and Wójcik-Fatla, A., “The occurrence of Toxoplasma gondii and Borrelia burgdorferi sensu lato in Ixodes ricinus ticks from eastern Poland with the use of PCR.” Annals of Agricultural and Environmental Medicine. 2009. 16(2): p. 313–319.
9. n.a., Draft Environmental Assessment: Florida Keys National Wildlife Refuges Complex Integrated Predator Management Plan. 2011, U.S. Fish & Wildlife Service: Big Pine Key, FL. http://www.fws.gov/nationalkeydeer/predatormgmt.html
10. n.a., “Around the Refuge System: Florida and Arizona.” Refuge Update. 2010. 2. p. 17.
11. Forys, E.A. and Humphrey, S.R., “Use of Population Viability Analysis to Evaluate Management Options for the Endangered Lower Keys Marsh Rabbit.” The Journal of Wildlife Management. 1999. 63(1): p. 251–260. http://www.jstor.org/stable/3802507

 North American Opossum with winter coat. Photo courtesy of
North American Opossum with winter coat. Photo courtesy of