Among the findings of a recent study:
Five of 18 cats trapped “between the spring and fall of 2008 and 2009” in central Illinois’ 1,500-acre Robert Allerton Park tested positive for Toxoplasma gondii antibodies. Five of the seropositive cats were trapped at the same site; there, one white-footed mouse (of 21 trapped) also tested positive, and a gray squirrel tested negative. The site where the sixth seropositive cat was trapped revealed similar results among the “small home range” (SHR) mammals found there: one of 34 white-footed mice was seropositive; a fox squirrel was negative.
All of which means… what, exactly?
Although there were five times as many “infected” cats at the first site, infection rates among SHR mammals were only about one-and-a-half times as high as those at the second site. Put another way: given the infection rate among SHR mammals at the second site, one would have expected three seropositive SHR mammals at the first site.
In fact, a press release put out last week put a very different spin on Shannon Fredebaugh’s thesis work (downloadable PDF):
One third of the cats sampled were infected with T gondii, as were significant numbers of the wild animals found at every site. Animals that inhabit or range over territories of 247 acres (100 hectares) or more, such as raccoons and opossums, were more likely to be infected than those with smaller ranges.
But these animals “could have acquired T. gondii infection somewhere outside of the park,” said Nohra Mateus-Pinilla, a wildlife veterinary epidemiologist at the University of Illinois Prairie Research Institute and leader of the study. Animals with smaller home ranges likely picked up the infection close to where they were trapped, she said. This makes these animals good sentinels of disease in a natural area. “The small animals are screening the environment for us,” she said. “So when we sample one of those animals, we are really sampling their lifestyle.”
The absence of bobcats in the park combined with the occurrence of domestic cats and T. gondii infection in wildlife that inhabit small territories strongly suggest that feral, free-ranging or abandoned house cats are the source of the infection, Mateus-Pinilla said. Cats are vital for the survival of the parasite, and so they are—either directly or indirectly—spreading T. gondii to the wildlife in the park. “There’s no other option,” she said.
Well, “one third of the cats” certainly sounds more impressive than “six of 18.” And “significant numbers of the wild animals found at every site” had an undeniable allure to it—though, in fact, the statement applies only to the park’s “large home range” (LHR) mammals (mostly raccoons and opossums).
Far more troubling, though, is the alleged connection between cats, T. gondii, and infected SHR mammals.
Environmental Contamination
“If one infected cat defecates there, any area can become infected,” Fredebaugh said in the press release. “It just takes one cat to bring disease to an area.”
But, as Fredebaugh points out, “environmental detection of oocysts is difficult and was not evaluated in this study.” [1] She simply assumes a causal link between “infected” cats and environmental contamination: more seropositive cats means more contaminated soil.
In fact, Fredebaugh goes further, assuming that the mere presence of cats—seropositive or not—is the key factor in SHR infection rates. In addition to trapping data, she uses data from scent stations and motion detection cameras (which proved largely ineffective, capturing photos of just four cats over the course of the research) to designate each of the eight sites as either high or low “cat occurrence,” as indicated in the following table (please forgive the tiny type):
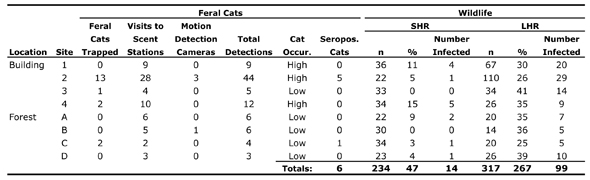
Fredebaugh acknowledges that “scent stations should only be used to identify trends in animal populations and as a supplemental tool in conjunction with other population estimates,” [1] thereby raising serious questions about their use in her study. (She’s not interested in trends, her scent station and trapping data correlate quite poorly, and her use of scent station data is hardly “supplemental.”)
But back to the environmental contamination.
Cats (both domestic and wild) are T. gondii’s definitive host—the animal in which the parasite reproduces sexually. Cats pass the mature, infective form of T. gondii in their feces—a process called “shedding oocysts.”
Although oocysts can survive in soil for up to 18 months, and are resistant to disinfectants, cats typically “shed oocysts only once in their life.” [see discussion in 2] Indeed, according to Dubey and Jones, “Most cats seroconvert after they have shed oocysts. Thus, it is a reasonable assumption that most seropositive cats have already shed oocysts.” [2]
So, who’s to say that the “infected” cats Fredebaugh trapped shed oocysts in the area where they were found? Indeed, we don’t even know that these cats shed oocysts in the park. It’s been suggested (based on a small sample of cats monitored closely from 1974 to 1977) that home ranges of unsterilized feral females can exceed 500 acres, while those of unsterilized feral males may approach 2,500 acres. (Even house-based males, which were also unsterilized, had large home ranges: 865–939 acres.) [3]
What’s more, Fredebaugh points out that, given their “relatively good physical condition,” some of these cats might have been “recently abandoned at RAP.” [1] In which case, they wouldn’t have been “contributing” any oocysts to the park’s soil—assuming they were seropositive to begin with.
Odds Ratios
Fredebaugh expresses her results using odds ratios, a measure easy enough to calculate but rather difficult to grasp intuitively (especially for those of us, myself included, unfamiliar with the measure). A page on the Children’s Mercy Hospital (Kansas City, MO) Website explains odd ratios this way:
“An odds ratio of 1 implies that the event is equally likely in both groups. An odds ratio greater than one implies that the event is more likely in the first group. An odds ratio less than one implies that the event is less likely in the first group.”
(Some examples are discussed in detail here.)
It seems to me that, in this case at least, odds ratios obscure more than they reveal. When Fredebaugh reports “a significant difference in the seroprevalence of T. gondii for SHR mammals at sites with a high frequency of cat occurrence,” we know nothing of sample size or the overall fit of the data (which, ranges from pretty good—for LHR mammals—to pretty lousy—for SHR mammals).
A simple x-y graph illustrates this point:
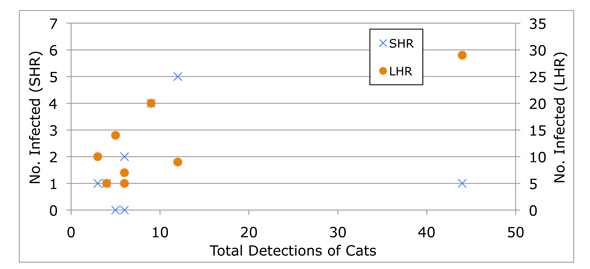
By (mis?)representing the data in odds ratios, Fredebaugh suggests a connection that’s not actually supported by her research findings.
That said, she’s is hardly the first to imply causation where nothing more than correlation has been demonstrated (and, again, even that is dicey). In “The Impact of Free Ranging Cats,” a special section of the Spring Issue of The Wildlife Professional, for example, David Jessup and Melissa Miller argue that “the science points to cats,” but provide little more than “proximity” and “sheer numbers” to support their claim that “outdoor pet and feral domestic cats may be the most important source of T. gondii oocysts in near-shore marine waters.” [4]
(No?) Other Options
The fact that the researchers are so certain of their conclusions—that the only explanation for T. gondii in Robert Allerton Park is the presence of cats—is telling. I can’t help but think that they knew going in what they would find (a perception reinforced by what’s included in, and omitted from, Fredebaugh’s literature review, as described below).
In fact, Mateus-Pinilla’s comment—“There’s no other option.”—is challenged by several recent studies.
“Among white-footed mice,” writes Fredebaugh, “I found a 6 percent seroprevalence of T. gondii antibodies, which was high, compared to other studies… Mice have a short life span, thus the findings that mice, including some juveniles, were seropositive implies an active infection and recent T. gondii contamination in RAP.” [1]
Actually, researchers at the University of Salford’s Centre for Parasitology and Disease Research found an overall prevalence of 59 percent among the “200 mice… trapped from within houses in the Cheetham Hill area of Manchester.” [5] More important, they observed “high levels of congenital transmission… with 75 percent of female mice transmitting parasites to foetuses prior to birth” (emphasis added), leading them to conclude:
“These high levels of congenital transmission in this wild population of mice, taken together with other recent data on congenital transmission in sheep, suggests that this phenomenon might be more widespread than previously thought.” [5]
Fredebaugh, by contrast, mentions congenital transmission only in passing.
In another paper, researchers from the Centre for Parasitology and Disease Research challenge the conventional wisdom surrounding the transmission of T. gondii (note: I’ve removed several in-text citations for the sake of readability):
“The life cycle is well understood and three principal routes are recognised: ingestion of infective oocysts shed by the cat, consumption of undercooked meat containing Toxoplasma cysts and congenital transmission. Traditionally, the main route of infection is considered to be infection by oocysts deposited in faeces by the definitive host, the cat. This would imply that a high degree of contact with cats would be required to explain the very high prevalences found in many animal and human populations. Toxoplasma gondii has been reported in a very wide range of species. However, this also includes some species that would not normally come into contact with cats.” [6]
“Congenital transmission,” suggest Hide et al., “offers another possible mode of parasite transmission in the absence of cats.” [6]
“One way of determining the importance of transmission routes is to investigate transmission in a system where one of the routes of transmission is absent or minimal. For example, the carnivorous route could be excluded as a source of transmission in a herbivorous species such as sheep.” [6]
On the basis of multiple studies (see [7] and [8] for details of the study with sheep), Hide and his colleagues make a compelling argument that congenital transmission “may be more important than previously considered.” [6]
Researchers working in “the remote, virtually cat-free, high arctic islands of Svalbard” (the northern-most part of Norway) [9] came to similar conclusions. Among the “arctic foxes (n = 594), Svalbard reindeer (n = 390), sibling voles (n = 361), walruses (n = 17), kittiwakes (n = 58), barnacle geese (n = 149), and glaucous gulls (n = 27),” tested, Prestrud et al. found T. gondii only in the arctic foxes (257, or 43 percent), geese (11, or 7 percent), and walruses (1, or 6 percent). [10]
“The finding of no seropositive reindeer or sibling voles,” they argue, “indicates that infection by oocysts is not an important mode of transmission on Svalbard.” [10] (Also of interest is their suggestion that the seropositive walrus demonstrates “that T. gondii is present in the marine food chain.” [10])
So where does the T. gondii come from?
“…we suggest that T. gondii most likely is brought to Svalbard by migratory birds that become infected in temperate agricultural areas in the winter. However, marine sources of infection may exist. The high seroprevalence of T. gondii in the arctic fox population on Svalbard may be due to: (1) infection from migratory bird species through predation; (2) vertical transmission; and (3) tissue cyst transmission within the Svalbard ecosystem through scavenging and cannibalism. Together, these transmission routes cause a surprisingly high seroprevalence of T. gondii in a top predator living in an ecosystem with very few cats.” [10]
A study of polar bears is further evidence that “other options” do indeed exist:
“In Svalbard cats are banned by the Norwegian authorities; however, a few cats may exist in Russian mining communities. Thus, the possibility of cats as a source of infection for polar bears cannot totally be excluded. Nonetheless, the existing cat population is very limited and local, and the proportion of seropositive polar bears is rather high, indicating that polar bears are commonly infected with T. gondii. It would, therefore, be inconceivable to assume that the few cats would play a major role in the epidemiology of T. gondii in the vast high Arctic. This is apparently the case in East Greenland as well.” [11]
As with the single seropositive walrus discussed above, the results of the polar bear study indicates “that there might be marine sources of T. gondii in the region.” [9]
And finally, in a paper published in 2009, Polish researchers proposed yet another possibility. The “high incidence of T. gondii found, among others, in free-living ruminants,” write Sroka et al., “suggests a possibility of other, so far unknown, paths of transmission of this protozoan.”
“Due to the fact that they are widespread, and tick-bites occur frequently both in humans and in animals, ticks might play an important role in toxoplasmosis transmission.” [12] (Note: the authors acknowledge both support for, and differing opinions about, the possibility of such a pathway.)
Fredebaugh mentions none of this work in her thesis; none of the author’s names appear in her lengthy list of references (which, to most people, probably appears comprehensive). And still, both she and Mateus-Pinilla (who chaired Fredebaugh’s thesis advisory committee) are committed to the proposition that, as Jessup and Miller suggest, “the science points to cats.”
Greater (Mis)Understanding
Fredebaugh concludes her thesis by suggesting that her results:
“provide a greater understanding of how feral cats and wildlife utilize natural areas in a highly fragmented landscape and how feral cat land use may impact wildlife parasite prevalence both directly and indirectly. With this information, I more clearly understand the association between wildlife and feral cats and can suggest better control strategies for feral cat populations. Using wildlife with small spatial scale habitat use as sentinels of parasite presence in the environment, I can gain a better understanding of the epidemiologic impact of T. gondii in different urban and rural settings to prevent human and wildlife infection. Further collaborative research is needed to determine the most effective management strategy for feral cat populations in natural areas and to evaluate the direct relationship between feral cats and their impacts on wildlife.” [1]
At the risk of being overly critical, I’m suggesting that Fredebaugh’s work has not only failed to clarify our understanding of feral cats, wildlife, and the transmission of T. gondii, but has—due to its problematic methodology and incomplete literature review—actually made matters worse (especially with regard to possible “control strategies”).
• • •
Not surprisingly, The Wildlife Society’s CEO/Executive Director Michael Hutchins immediately endorsed the study (his summary conveniently omits the small sample size involved, the inverse relationship between “infected” cats and “infected” SHR mammals, and several other important aspects of the research) and its misguided conclusions, pleading:
“How many more peer reviewed studies do we need to convince leaders to change the way that we are currently dealing with the feral cat population explosion in this country?”
I don’t want to suggest that Hutchins and I are on the same page here, but omit the word explosion, and that’s pretty much the same question I’ve been asking for a while now.
Literature Cited
1. Fredebaugh, S.L., Habitat Overlap and Seroprevalence of Toxoplasma Gondii in Wildlife and Feral Cats in a Natural Area. 2010, University of Illinois at Urbana-Champaign: Urbana-Champaign, IL. p. 88. http://www.ideals.illinois.edu/bitstream/handle/2142/16185/1_Fredebaugh_Shannon.pdf?sequence=6
2. Dubey, J.P. and Jones, J.L., “Toxoplasma gondii infection in humans and animals in the United States.” International Journal for Parasitology. 2008. 38(11): p. 1257–1278. http://www.sciencedirect.com/science/article/B6T7F-4S85DPK-1/2/2a1f9e590e7c7ec35d1072e06b2fa99d
3. Liberg, O., “Home range and territoriality in free-ranging house cats.” Acta Zoologica Fennica. 1984. 171: p. 283–285.
4. Jessup, D.A. and Miller, M.A., “The Trickle-Down Effect.” The Wildlife Professional. 2011. 5(1): p. 62–64.
5. Marshall, P.A., et al., “Detection of high levels of congenital transmission of Toxoplasma gondii in natural urban populations of Mus domesticus.” Parasitology. 2004. 128(01): p. 39–42. http://dx.doi.org/10.1017/S0031182003004189
6. Hide, G., et al., “Evidence for high levels of vertical transmission in Toxoplasma gondii.” Parasitology. 2009. 136(Special Issue 14): p. 1877-1885. http://dx.doi.org/10.1017/S0031182009990941
7. Morley, E.K., et al., “Significant familial differences in the frequency of abortion and Toxoplasma gondii infection within a flock of Charollais sheep.” Parasitology. 2005. 131(02): p. 181–185. http://dx.doi.org/10.1017/S0031182005007614
8. Morley, E.K., et al., “Evidence that primary infection of Charollais sheep with Toxoplasma gondii may not prevent foetal infection and abortion in subsequent lambings.” Parasitology. 2008. 135(02): p. 169–173. http://dx.doi.org/10.1017/S0031182007003721
9. Prestrud, K.W., et al., “Direct high-resolution genotyping of Toxoplasma gondii in arctic foxes (Vulpes lagopus) in the remote arctic Svalbard archipelago reveals widespread clonal Type II lineage.” Veterinary Parasitology. 2008. 158(1-2): p. 121–128. http://www.sciencedirect.com/science/article/B6TD7-4TDK6Y8-2/2/1e5b02861f7a0c81f2277f65f42e6be9
10. Prestrud, K.W., et al., “Serosurvey for Toxoplasma gondii in arctic foxes and possible sources of infection in the high Arctic of Svalbard.” Veterinary Parasitology. 2007. 150(1-2): p. 6–12. http://www.sciencedirect.com/science/article/B6TD7-4PYR4P2-2/2/fcc91fcf1d1426cd1b750bd3840bdb31
11. Oksanen, A., et al., “Prevalence of Antibodies Against Toxoplasma gondii in Polar Bears (Ursus maritimus) From Svalbard and East Greenland.” Journal of Parasitology. 2009. 95(1): p. 89–94. http://dx.doi.org/10.1645/GE-1590.1
12. Sroka, J., Szymańska, J., and Wójcik-Fatla, A., “The occurrence of Toxoplasma gondii and Borrelia burgdorferi sensu lato in Ixodes ricinus ticks from eastern Poland with the use of PCR.” Annals of Agricultural and Environmental Medicine. 2009. 16(2): p. 313–319.